In a breakthrough for metastatic breast cancer treatment, researchers from Sun Yat-sen Memorial Hospital, Sun Yat-sen University, have shown that combining pyrotinib, an irreversible tyrosine kinase inhibitor, with fulvestrant, a selective estrogen receptor degrader, delivers robust clinical benefits for HR+/HER2+ metastatic breast cancer patients.
The phase II trial, conducted across five hospital in China, included 46 patients who had previously experienced progression on trastuzumab-based therapies. Participants received daily oral pyrotinib (400 mg) and fulvestrant injections (500 mg, administered per protocol). The study aimed to evaluate the efficacy and safety of this novel combination.
Key Findings:
Progression-Free Survival (PFS): Median PFS reached 18.2 months across all participants, with first-line patients achieving 19.5 months. Even in patients with brain metastases—a subgroup notoriously challenging to treat—the PFS was 18.4 months.
Disease Control Rate (DCR): An impressive 97.5% of patients exhibited disease control, with 32.5% achieving objective responses, including complete and partial responses.
Safety Profile: The treatment was well tolerated, with no grade 4 or higher adverse events reported. Diarrhea was the most common grade 3 adverse event (26.1%), managed effectively with supportive care.
The potential for precision medicine : The study also explored biomarkers to predict treatment response. Patients with low tumor mutation burden (TMB) and ZNF217 mutations exhibited enhanced responsiveness, underscoring the potential for precision medicine applications.
This trial highlights pyrotinib and fulvestrant as a promising chemotherapy-free alternative for HR+/HER2+ metastatic breast cancer, especially for patients resistant to trastuzumab or those with brain metastases. Its favorable safety profile and strong efficacy data position it as a potential first-line treatment in future clinical practice.
Building on these promising results, the research team plans to initiate a phase III randomized controlled trial comparing this regimen against standard first-line therapies. Advanced technologies, including artificial intelligence, will be leveraged to optimize patient selection and monitor trial outcomes in real time.
See the article: https://doi.org/10.1002/mco2.70031
MedComm
10.1002/mco2.70031
Combined pyrotinib and fulvestrant for hormone receptor-positive and HER2-positive metastatic breast cancer: A multicenter, single-arm, phase II trial
20-Dec-2024
Additional Media
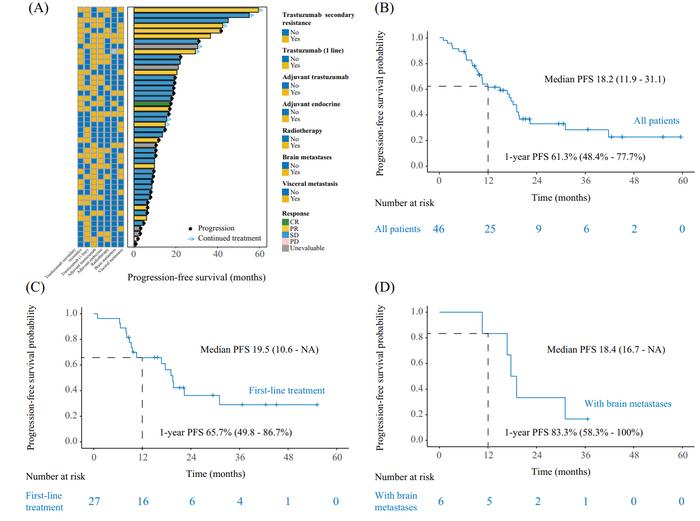
In the overall study population, the median progression-free survival (PFS) in the combination therapy group was 18.2 months (95% CI: 11.9–31.1). This result highlights the effectiveness of pyrotinib and fulvestrant in controlling tumor progression in the challenging context of HR+/HER2+ metastatic breast cancer. Notably, the median PFS for patients receiving the combination as first-line therapy was 19.5 months, demonstrating robust efficacy in those undergoing initial treatment. Furthermore, f Credit: Ying Wang
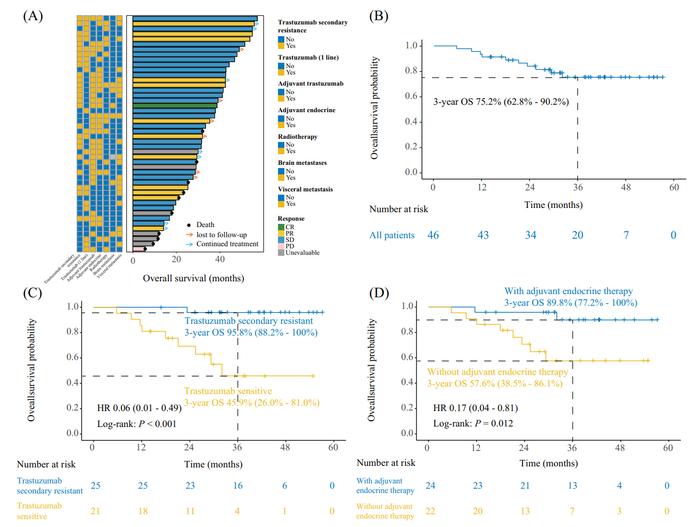
The study revealed significant overall survival (OS) benefits with the combination of pyrotinib and fulvestrant for HR+/HER2+ metastatic breast cancer patients. Notably, trastuzumab-resistant patients achieved an exceptional 3-year survival rate of 95.8% (95% CI: 88.2–100%). Furthermore, patients receiving prior endocrine therapy had a 3-year survival rate of 89.8%, highlighting the potential of this combination therapy in extending survival for specific patient subgroups. Credit: Ying Wang
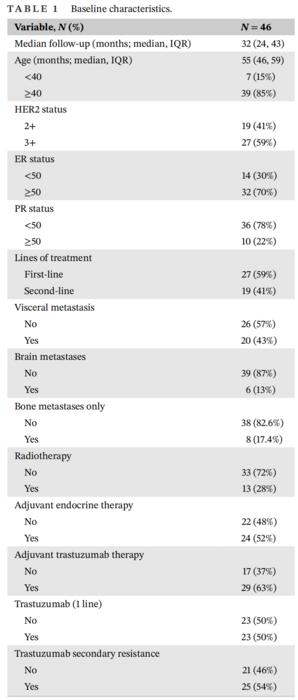
The baseline characteristics of the 46 patients enrolled in this study included a median age of 55 years (IQR: 46–59), with 85% aged 40 or older. Among the patients, 59% had HER2 3+ status, and 41% had HER2 2+ confirmed through FISH testing. Estrogen receptor (ER) expression was ≥50% in 70% of patients, while 78% had progesterone receptor (PR) expression Credit: Ying Wang
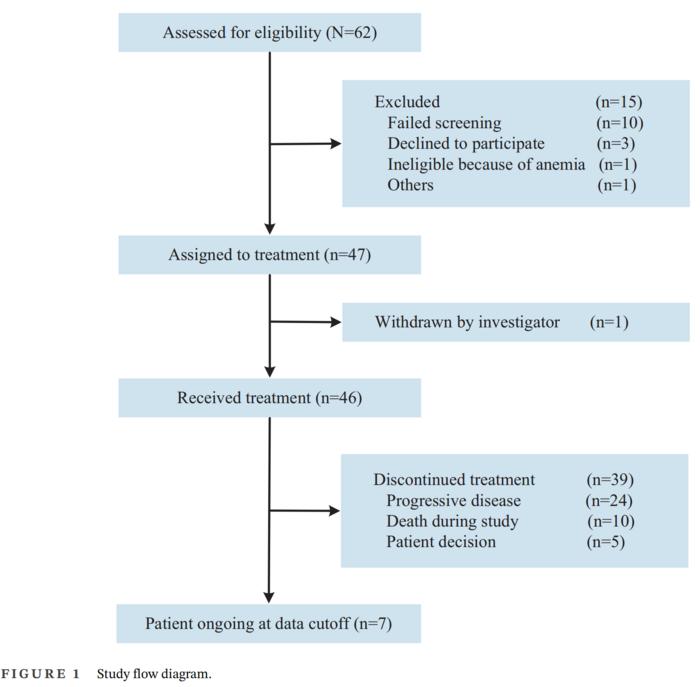
A total of 62 patients were initially screened across five study centers for eligibility in this study. Of these, 15 patients were excluded due to various reasons: 10 failed the screening, 3 declined participation, 1 was ineligible due to anemia, and 1 was excluded for other reasons. Ultimately, 46 patients met the eligibility criteria and were enrolled to receive the combination treatment of pyrotinib (400 mg orally once daily) and fulvestrant (500 mg intramuscularly, per protocol). These patie Credit: Ying Wang
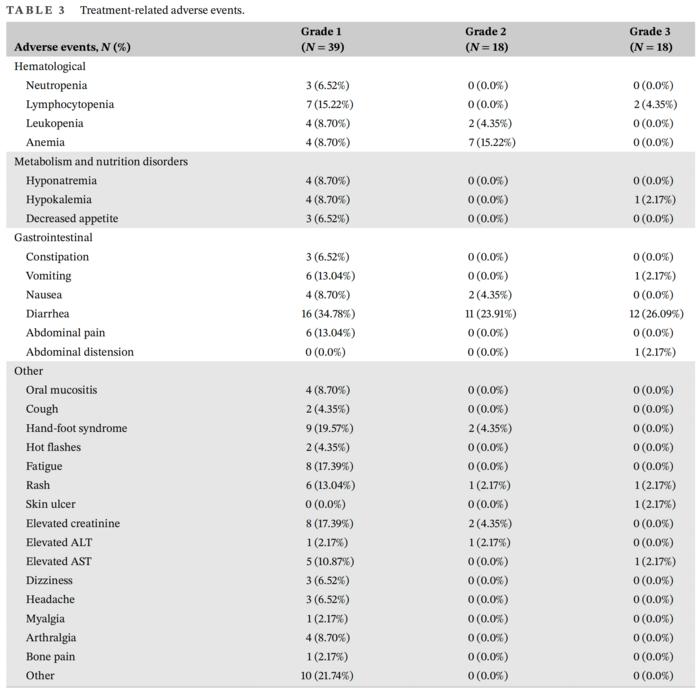
In terms of safety, the combination of pyrrolizinib and fulvestrant was well tolerated. No grade 4 adverse events or treatment-related deaths occurred, although adverse effects of grade 3 or higher were reported in 43.5% of the patients. The most common grade 3 adverse event was diarrhea (26.1%), which was not seriously affected by dose modification or symptomatic management (e.g., antidiarrheal drugs). This combination therapy was associated with less severe side effects than conventional chemo Credit: Ying Wang
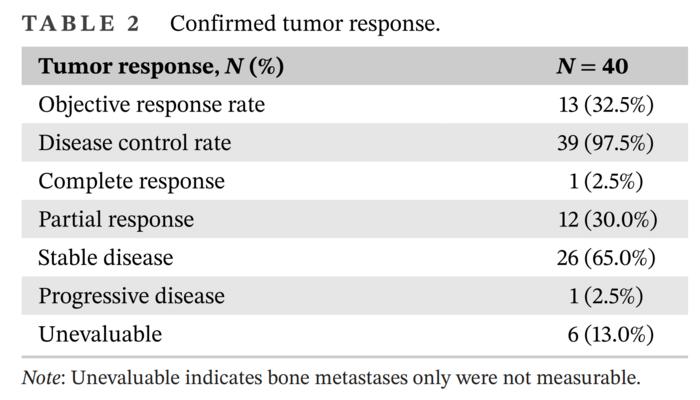
The high DCR(97.5%) and ORR(32.5%) indicate that this combination regimen has a superior clinical effect in the management of HR+/HER2+ metastatic breast cancer, and it is an important alternative treatment option especially for patients who cannot tolerate conventional chemotherapy. Credit: Ying Wang